We are now into month eleven of COVID-19 vaccinations globally, and eight months into the Australian inoculation process, 4 weeks since our last update.
| Date | Doses Administered (millions) | Countries | Run rate (millions per day) |
| 22-Feb | 186 | 82 | 6.34 |
| 22-Mar | 410 | 132 | 9.96 |
| 19-Apr | 848 | 154 | 17.9 |
| 17-May | 1,380 | 176 | 22.5 |
| 16-Jun | 2,420 | 180 | 31.1 |
| 3-Aug | 4,160 | 180 | 42.0 |
| 3-Sep | 5,390 | 183 | 41.0 |
| 7-Oct | 6,410 | 184 | 28.7 |
| 8-Nov | 7,260 | 184 | 31.3 |
Source: Mason Stevens, Our World in Data
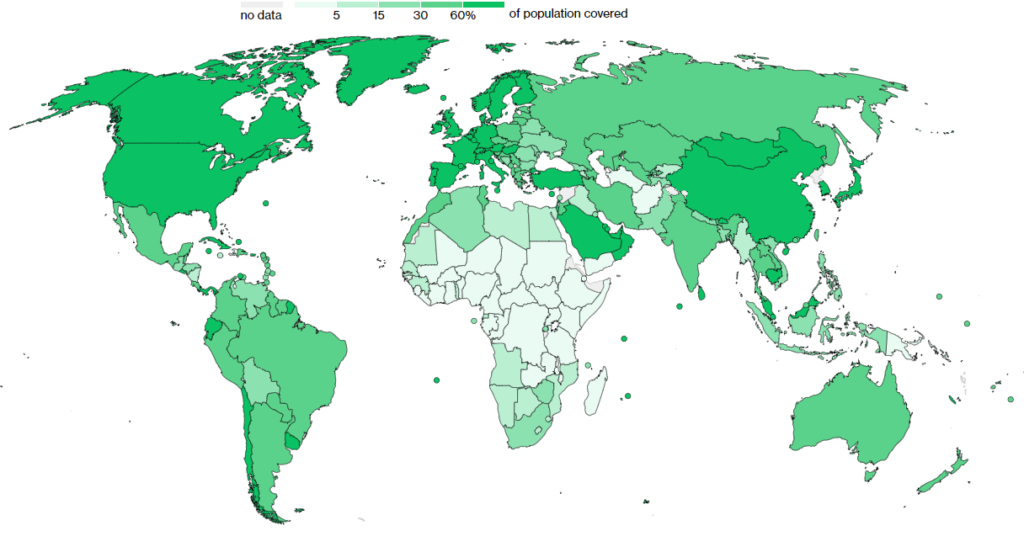
That’s enough doses to provide at least 1 dose to 94% of the global population
Visually, the laggard nations tend to be the poorer nations who were slower to secure vaccine supply, but also had limited ability to rollout vaccines requiring cold temperatures to remain viable.
Wealthy nations have vaccinated 15-20x faster than these poorer nations, which we can see from the lighter green colouring in parts of southeast Asia, Africa and South America.
Of this, the least wealthy 52 nations have 4.7% of all vaccinations but have 20.5% of the world’s population.
Source: Bloomberg
The Development of COVID-19 Pills
There are currently two promising candidates for treating COVID-19 patients early in the course of the virus.
Last month, Merck and their partner Ridgeback Biotherapeutics submitted their experimental pill to regulators after a study showed it slashed the risk of getting seriously ill or dying by half in patients with mild-to-moderate COVID-19.
Then on Friday, Pfizer announced that its COVID-19 pill reduced hospitalisations and deaths in high-risk patients by 89%, a result that could possibly alter the way the coronavirus is treated.
In a statement on Friday, Pfizer said it was no longer taking new patients “due to overwhelming efficacy” and planned to submit the findings to US regulatory authorities, seeking emergency authorisation.
Pfizer also published some details of the trial – which we note have not been published in a medical journal yet, and as such haven’t been verified – having tested the pill on 1,219 unvaccinated adults, where five days of treatment (using the pill) reduced the rate of hospitalisation within three to five days of symptom onset.
The drug, Paxlovid, binds to an enzyme called a “protease” to stop the virus from replicating itself, a similar way to how HIV medications can work.
In Merck’s statement last month, the company mentioned that the need for a pill form treatment is so acute that they’d already agreed to allow generic drugmakers to apply for licenses to make its treatment available in more than 100 low-middle income nations, allowing greater access worldwide.
The UK’s drug regulators became the first country in the world to greenlight Merk’s drug last Thursday, after a swift review.
Australia’s Vaccination Progress
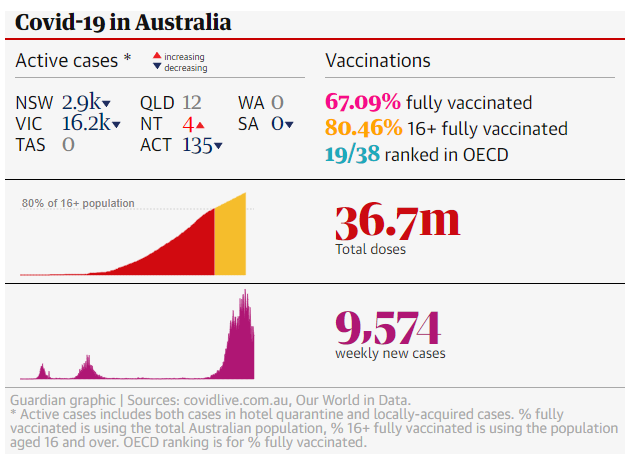
In Australia, we’ve continued to vaccinate quickly as 67.09% of Australian residents are now fully vaccinated.
Our ability to maintain our vaccination pace and vaccinate a higher portion of our adult population has seen us improve in the OECD coverage rankings, from 31/38 to 19/38 (mid field).
As at 5-November, we’ve given 36.7 million jabs, an increase of 7.4 million jabs month-on-month, and ~20% more of our total population vaccinated.
Reopening Australia
Over the past eight months that we’ve been vaccinating Australian residents, our targets have changed dramatically from “it’s not a race” when we fell far short of our 4 million vaccinations by March, and never got close to the whole country being vaccinated by October.
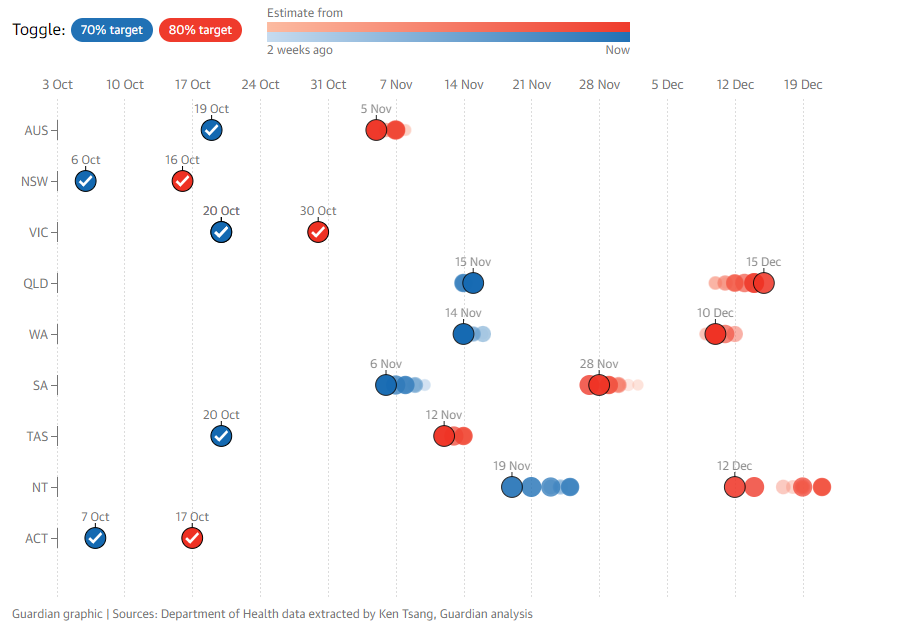
The most recent plan, dubbed “Operation Covid Shield”, suggested we aim for 80% of the 16+ aged population to be vaccinated by December.
The Federal government also set double dose vaccination targets of 70% and 80% for 16+ as the thresholds for Phase B and Phase C of its “National Plan to transition Australia’s National COVID-19 Response”, especially in respect to reducing lockdowns and opening up borders.
As such, we’ve reached the 80% target level for 16+ aged persons over the weekend, where there is quite a dispersion among states.
NSW, VIC and the ACT were first to reach the threshold, and as they account for >50% of Australia’s population, were the critical mass required for the average to reach 80%+.
QLD, WA and NT are still some ways off as of yet to reaching the threshold, likely reaching it in December, whereas TAS and SA should reach the milestone in November.
As we’ve mentioned in previous missives on the subject, the Australian Technical Advisory Group on Immunisation (ATAGI) is now recommending vaccinating against COVID-19 for all individuals from 12 years of age, though as a lesser priority to older Australians.
There are 1.2 million people aged 12-15 in Australia, where adding them to the rollout in September only minimally slowed down our overall rollout by a week.
If ATAGI were able to add 5–11-year-olds at a later date, that would add an additional 2.3 million Australians.
In aggregate, 5–15-year-olds would add 3.5 million people to the rollout, requiring 7 million doses of vaccines, which should still be within our expected 2021 mRNA supply, where all of these minors would be able to receive either Pfizer or Moderna.
In terms of vaccinating 5-11 year olds, Pfizer and their partner BioNTech SE are currently trialling if their COVID-19 vaccine is safe for 5-11 year olds, in a large-scale trial.
In the trial data that we saw in September, 2,268 participants (kids) received a 10mcg dose – about 1/3 of the dose 16–25-year-olds receive.
Pfizer were expected to seek US FDA emergency approval this month, whilst also sharing data with European health regulators too.
As of yet, I’m yet to hear if our local TGA is reviewing the data, but all-in-all it sounds promising.
Vaccination Hesitancy
The remaining factor in our vaccine rollout that we should discuss is those that are hesitant or firmly against vaccination, where they have the ability to receive a vaccine but choose not to.
Obviously, we’d want this group to be less than 20% of our population, to allow us to hit the 80% 16+ threshold.
Ideally, we’d want it to be as minute a proportion of the population as possible, minimising the chance of widespread hospitalisations occurring.
This will have to be an evolving dynamic, as well, as vaccine hesitancy should hopefully translate well to people keeping their antibodies up to date through either attaining a third dose of the available vaccines or being willing to use the Merck or Pfizer pill-form supplements, when approved and when available.
In terms of measuring vaccine hesitancy in Australia, the best data source I’ve found is produced by the Melbourne Institute, part of Melbourne University.
Their Vaccination Report (see hyperlink) shows that as at 21-October (latest report) total vaccine hesitancy amongst our adult population was 11.8%, down from ~15% on 23-September.
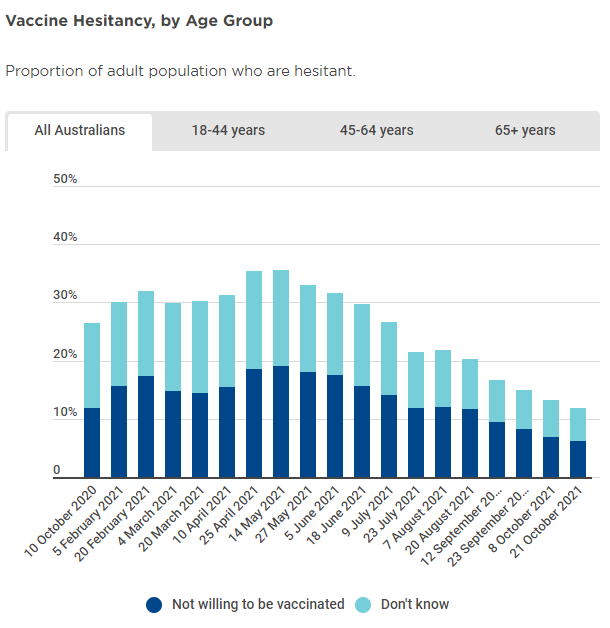
Source: Melbourne Institute
On a state-by-state basis, hesitancy is fairly well distributed across the nation, though SA, WA and QLD have slightly above average hesitancy levels, which may be a key reason why they haven’t yet reached the 80% 16+ threshold.
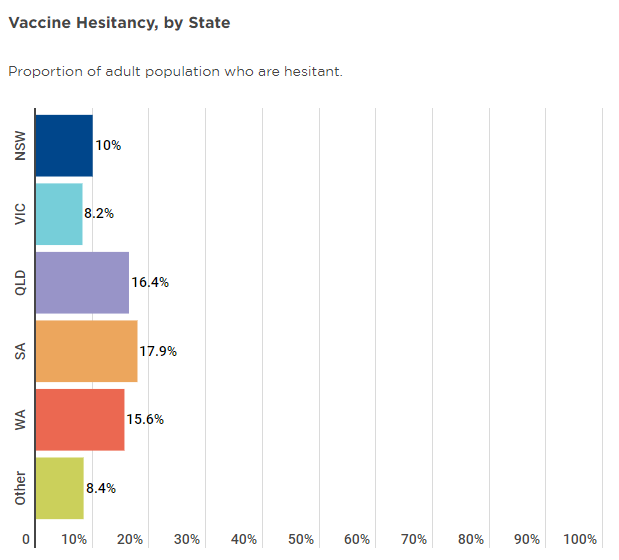
Source: Melbourne Institute
Closing Remarks
The COVID-19 pills being developed by Merck and Pfizer are strongly positive developments, as they allow a greater degree of normalcy to return to most people’s lives, and that they’re being made available to poorer nations as well who have been relatively left behind in the vaccination roll-out.
They also cross a line of demarcation, where this pandemic transforms into an endemic, where some variant of the coronavirus is likely to affect a small portion of the population, years into the future.
We may really now be “living with the virus”, but we’re also getting better and better armed in dealing with it.
The views expressed in this article are the views of the stated author as at the date published and are subject to change based on markets and other conditions. Past performance is not a reliable indicator of future performance. Mason Stevens is only providing general advice in providing this information. You should consider this information, along with all your other investments and strategies when assessing the appropriateness of the information to your individual circumstances. Mason Stevens and its associates and their respective directors and other staff each declare that they may hold interests in securities and/or earn fees or other benefits from transactions arising as a result of information contained in this article.